Bullous Pemphigoid vs. Pemphigus Foliaceus vs. Pemphigus Vulgaris and Microbiome Relationship: Diagnosis, Support, Resolution, and Testimonial
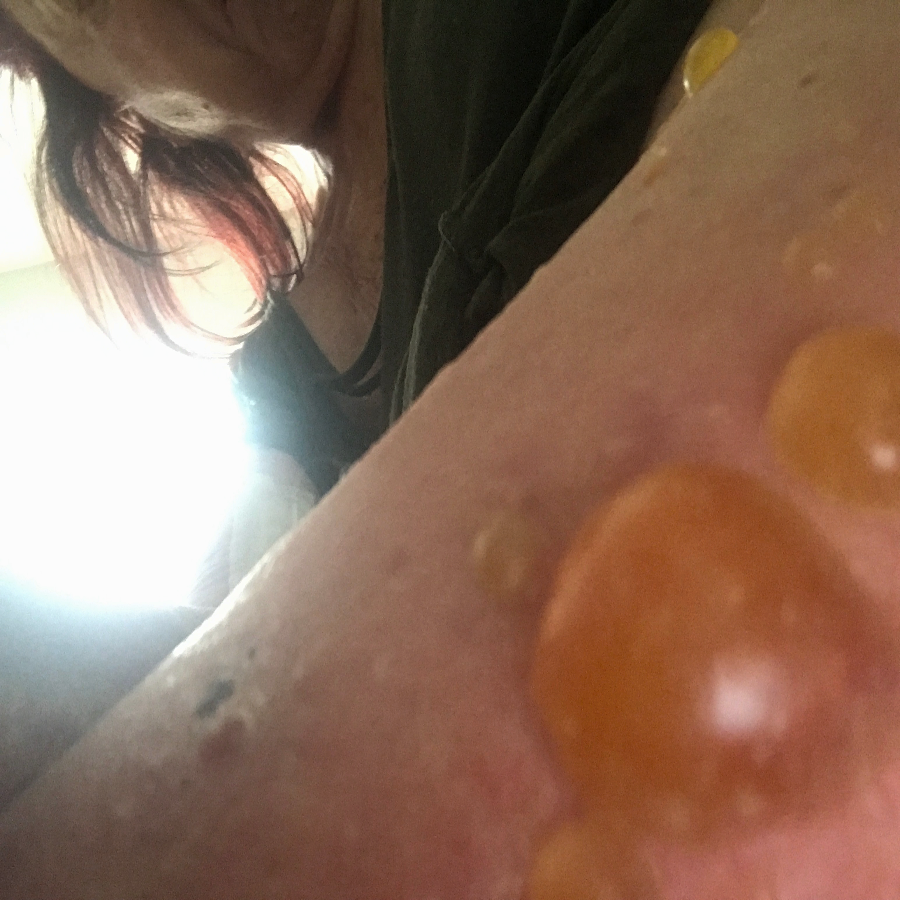
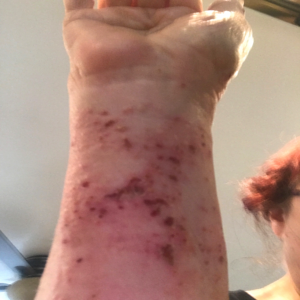
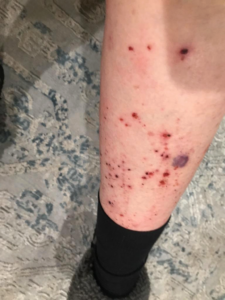
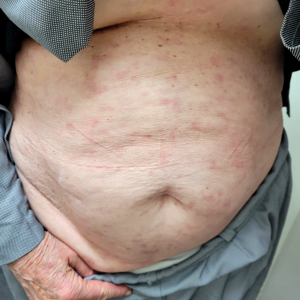
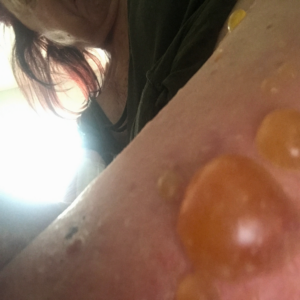
Patient: A 77-year-old female experiencing pain and fluid-filled blisters all over her body and inside of her mouth, as well as severe emotional distress. She was initially worked up on 7-30-24 by her HMO, and the diagnosis was not 100% conclusive. The Key Differences in Diagnostic Criteria for Pemphigus Foliaceus (PF), Pemphigus Vulgaris (PV), and Bullous Pemphigoid (BP) can be found in the table below. The positive finding for the patient will be in RED. The final diagnosis based on the immunofluorescence microscopy report was BULLOUS PEMPHIGOID, even though the physical examination found blisters in the mouth, superficial, scaly, crusted erosions, and elevated Desmoglein 1 ABs. Based on all findings, the patient had criteria that fit all three diagnoses. Additional blood tests showed elevated mean corpuscular volume (MCV), hematocrit, low hemoglobin, and RBC count. This is typically classified as macrocytic anemia, with the most common causes being B12 and/or folate deficiencies. As far as I was concerned, the diagnosis was irrelevant because the underlying cause would be the same, and my supportive recommendations would be the same.
The initial medical treatment was doxycycline, high-dose niacin (B3), and hydroxyzine (an antihistamine). This had no effect. The patient was then placed on a high-dose prednisone-taper protocol. The medication caused 20 lbs weight gain, extreme edema in the lower extremities, and severe itching. The water retention eventually warranted the need for a diuretic to be taken. The Patient was unable to continue the prednisone due to unwanted effects. Also, the blisters were not responding to the medications. This is when the patient was referred to me for a second opinion.
A thorough consultation and record review were completed. A supportive protocol, including lifestyle and dietary modifications and targeted nutraceuticals, was recommended. Being a seasoned gut health expert, her dietary modifications were the most significant change, as her prior diet consisted of frozen meals and eating out. Based on 36 years of clinical nutrition, her nutritional supplement protocol focused on supporting oxidative stress within the mitochondria and endoplasmic reticulum.
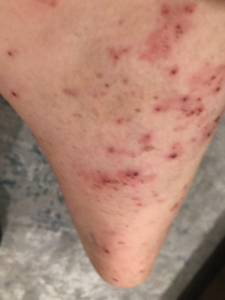
The patient was seen for four visits over three and a half months. By the third visit, complete resolution was achieved without pharmaceutical intervention. The final visit was to confirm a durable resolution.
Pemphigus Vulgaris and Bullous Pemphigoid: Overview, Similarities, Differences, and Microbiome Correlation
What Are Pemphigus Vulgaris and Bullous Pemphigoid?
Both pemphigus vulgaris (PV) and bullous pemphigoid (BP) are autoimmune blistering diseases, but their underlying mechanisms, severity, and clinical presentation differ.
Pemphigus Vulgaris (PV) is caused by autoantibodies targeting desmoglein 1 and 3, proteins that help skin cells adhere to each other. This leads to intraepidermal blisters, primarily affecting mucous membranes and skin. The blisters are fragile, rupture easily, and leave painful erosions.
Bullous Pemphigoid (BP) occurs when autoantibodies attack BP180 and BP230, basement membrane components anchoring the epidermis to the dermis. This causes subepidermal blisters, which are tense, fluid-filled, and less prone to rupture. BP mainly affects the skin, sparing mucous membranes in most cases.
Initial Presentation
Similarities Between PV and BP
- Autoimmune origin: Both involve the immune system attacking structural skin proteins.
- Chronic nature: Both require long-term management and can relapse.
- Blister formation: Both cause skin blistering, though PV blisters are more fragile.
- Treatment: Both are treated with corticosteroids, immunosuppressants, and biologic therapies like rituximab.
- Potential triggers: Both have been associated with drugs, infections, and environmental factors.
Key Differences in Diagnostic Criteria (Patient Criteria Red)
| Feature | Pemphigus Foliaceus (PF) | Pemphigus Vulgaris (PV) | Bullous Pemphigoid (BP) |
|---|---|---|---|
| Blister Type | Superficial, scaly, crusted erosions | Flaccid blisters, easily ruptured, leading to painful erosions | Tense, fluid-filled blisters that do not rupture easily |
| Primary Location | Skin (seborrheic areas like the scalp, face, and upper trunk); spares mucosa | Mucous membranes (mouth, throat) and skin | Skin (trunk, arms, legs); mucosal involvement is rare |
| Nikolsky’s Sign | Positive (skin shears off with slight pressure) | Positive (gentle pressure causes skin to slough off) | Negative or weakly positive |
| Histopathology | Subcorneal acantholysis (loss of adhesion in the upper epidermis) | Intraepidermal acantholysis (loss of adhesion in the entire epidermis) | Subepidermal blistering (blister forms between epidermis and dermis) |
| Direct Immunofluorescence (DIF) | IgG and C3 deposits in the upper epidermis, “chicken wire” pattern | IgG and C3 deposits in the entire epidermis, “chicken wire” or “honeycomb” pattern | IgG and C3 deposits in the basement membrane zone, linear pattern |
| Indirect Immunofluorescence (IIF) | Circulating anti-desmoglein 1 antibodies | Circulating anti-desmoglein 1 and 3 antibodies | Circulating anti-BP180 and BP230 antibodies |
| Enzyme-Linked Immunosorbent Assay (ELISA) | Elevated anti-desmoglein 1 antibodies | Elevated anti-desmoglein 1 and 3 antibodies | Elevated anti-BP180 and BP230 antibodies |
Is There a Correlation Between Pemphigoid, Pemphigus, and Microbiome?
Recent research highlights a potential link between the gut microbiome and autoimmune skin diseases like pemphigus vulgaris (PV) and bullous pemphigoid (BP). Below is a detailed breakdown of how gut dysbiosis, intestinal permeability, and specific microbial interactions may contribute to the pathogenesis of these conditions.
1. Dysbiosis and Immune Dysregulation
Dysbiosis, an imbalance in gut microbiota, is increasingly recognized as a contributor to autoimmune diseases, including PV and BP. The gut microbiome plays a crucial role in immune system modulation, and disruptions in microbial composition can trigger systemic inflammation and promote autoantibody production. In fact, BP180 and BP230 are both proven to be expressed in the intestinal epithelium. BP was closely related to inflammatory bowel disease (IBD), which has been proven to be induced by gut microbiota dysbiosis.
Microbiota Alterations in PV and BP
Reduced microbial diversity: Patients with autoimmune diseases often exhibit lower gut microbiome diversity, associated with a weakened ability to regulate immune responses. There are subtle differences in microbiome presentation between PV and BP. Still, the general theme is decreased SCFA-producing and anti-inflammatory bacteria and an abundance of inflammatory, gram-negative bacteria.
Loss of beneficial bacteria: Studies suggest that PV and BP patients have reduced Lactobacillus and Bifidobacterium, both known for their anti-inflammatory and immune-regulating effects.
Increase in pro-inflammatory bacteria: Some pro-inflammatory bacteria, such as Escherichia coli, Bacteroides fragilis, and Clostridium species, are more abundant in PV and BP patients, promoting systemic inflammation.
Altered short-chain fatty acid (SCFA) production: Decreased levels of healthy gut bacteria, such as Faecalibacterium prausnitzii and Akkermansia muciniphila, which produce SCFAs (butyrate, acetate, propionate), help regulate T-cell function, inhibit gut permeability, and suppress autoimmunity.
2. Intestinal Permeability (Leaky Gut) and Autoimmune Skin Diseases
A compromised intestinal barrier, commonly called “leaky gut,” allows undigested food particles, toxins, and microbial components (e.g., lipopolysaccharides, LPS) to enter the bloodstream. This systemic exposure to immune triggers can lead to chronic inflammation and the development of autoantibodies.
Supporting Pemphigoid, Pemphigus, and Microbiome, Especially Leaky Gut Mechanisms
Zonulin Dysregulation: Zonulin, a protein that regulates intestinal permeability, is upregulated in autoimmune diseases. This increases gut barrier permeability and allows immune-activating molecules to enter circulation.
Lipopolysaccharides (LPS) and Autoimmunity: LPS, a bacterial endotoxin found in Gram-negative bacteria, can induce systemic inflammation and disrupt the delicate balance of immune tolerance. Studies have demonstrated that LPS exposure increases the production of autoantibodies, which could exacerbate pemphigus and bullous pemphigoid.
Molecular Mimicry: Bacterial proteins and antigens can resemble desmogleins (in PV) or BP antigens (in BP). The immune system may mistakenly attack skin proteins after encountering similar bacterial components, triggering autoimmune skin reactions.
3. Specific Associations in Pemphigoid, Pemphigus and Microbiome
Certain microbial species have been linked explicitly to pemphigus vulgaris and bullous pemphigoid, suggesting that gut and skin microbiota influence disease onset and progression.
Pemphigus Vulgaris (PV)
Helicobacter pylori: Multiple studies suggest that H. pylori infection is more prevalent in PV patients. This bacterium can induce peptic ulcer, chronic inflammation, and gastritis while stimulating autoantibody production and increasing gut permeability.
In some cases, eradicating H. pylori in PV patients has been reported to reduce disease severity.
Porphyromonas gingivalis (Oral Microbiome Connection):P. gingivalis, a periodontal pathogen, has been linked to increased desmoglein-targeting antibodies. Chronic gum infections may act as persistent bacterial reservoirs and immune triggers, exacerbating pemphigus symptoms.
Bullous Pemphigoid (BP)
Escherichia coli & Clostridium difficile: BP patients often show increased gut colonization by pro-inflammatory bacteria, including E. coli and C. difficile. These bacteria produce toxins that activate immune responses, potentially worsening BP symptoms.
Skin Microbiome Disruptions: BP patients often have altered skin microbiota, with reduced beneficial commensal bacteria (such as Cutibacterium) and increased Staphylococcus aureus, which promotes skin inflammation and autoimmune responses.
4. Digestion Coaching Recommendations Focused on the Therapeutic Potential of Probiotics and Prebiotics
Since dysbiosis and leaky gut contribute to immune dysregulation, modulating the gut microbiome through probiotics, prebiotics, and dietary interventions may help reduce the severity of autoimmune skin disease.
Probiotics and Their Potential Role in Supporting Pemphigoid, Pemphigus and Microbiome
Lactobacillus rhamnosus & Bifidobacterium breve: These probiotics enhance gut barrier function and reduce systemic inflammation. Studies in autoimmune conditions suggest they help regulate T-cell responses and reduce autoantibody production.
Saccharomyces boulardii: This yeast probiotic can neutralize gut-derived toxins and modulate immune activity. It has been studied for its anti-inflammatory effects in autoimmune conditions.
Prebiotics and Their Potential Role in Supporting Pemphigoid, Pemphigus and Microbiome
Inulin and Fructooligosaccharides (FOS): Both are types of polysaccharides, a group of complex carbohydrates, and they are both considered soluble dietary fibers. They feed beneficial gut bacteria and promote SCFA production.
Increased SCFA levels can help suppress overactive immune responses in PV and BP. Black Gram (Vigna mungo), green peas, and garbanzo beans with curry spices are perfect for this task.
Resistant Starch: In foods like cooked/cooled potatoes/rice, resistant starch enhances butyrate production, supporting gut barrier integrity and immune balance.
Conclusion:
It took 3 1/2 months from the initial consultation to the final picture. The patient complied with all recommendations, including lifestyle, dietary, and gut health modifications, targeted nutraceuticals, and routine diagnostic testing. What you are seeing in the final picture is post-traumatic hyperpigmentation. With routine sun exposure, it should all be resolved by the end of Summer.
COMPLEMENTARY 15-MINUTE CALL
Take your first step toward a renewed sense of well-being. Call today to arrange a complimentary 15-minute consultation.
Let’s discern whether my approach aligns with your needs.
I look forward to connecting with you at 714-639-4360.