I propose that the gut-brain and gut-mitochondria axes are not isolated systems but part of a deeply interconnected network, linked at the molecular level by microtubules, chemical messengers, and bioelectrical signals. This network, encompassing the gut-mitochondria-brain axis, responds to internal and external stimuli—or “cues”—that either align the body with its natural design, fostering health, or pull it toward disease and decay. The optimal functioning of this axis, as well as disruptions such as dysbiosis, mitochondrial oxidative stress, or reduced ATP production, serves as a bridge between the gut, energy production, and brain health. For this analysis, we’ll explore how dysbiosis-induced reductions in butyrate impair mitochondrial function in intestinal epithelial cells (IECs) and neurons, disrupting gut-brain signaling and contributing to conditions like depression, anxiety, autism spectrum disorder (ASD), and neurodegenerative diseases. Ultimately, total body optimization requires direct interaction with the natural world, through sunlight, diet, water, movement, and rest, to support this axis and maintain health by design.
The Gut-Mitochondria-Brain Axis: A Key to Mental, Neurological, and Whole-Body Health
What if the secret to vibrant physical, mental, and neurological health lies in the intricate interplay of your gut, mitochondria, and brain, all connected at the molecular level? Every component of our body—from cells to molecules—is linked through microtubules, chemical messengers like short-chain fatty acids (SCFAs), and bioelectrical signals that transmit information across tissues. This interconnectedness is orchestrated by a network of gut-body axes, including the gut-mitochondria-brain axis, which integrates cues from within the body (e.g., microbial metabolites) and from the external environment (e.g., sunlight, diet). When these cues align with our natural design, they promote health; when they are disrupted, they drive disease. Emerging research reveals that the gut microbiome, mitochondria (the cellular powerhouses), and brain (the hub of cognition and emotion) form a dynamic triad. Disruptions like microbial imbalances (dysbiosis), mitochondrial stress, or impaired brain signaling create a feedback loop, initiating or exacerbating conditions like depression, anxiety, ASD, and neurodegenerative diseases.
The Gut-Mitochondria-Brain Axis: A Unified Network
The gut-mitochondria-brain axis brings together the gut-brain axis (two-way communication between your digestive system and brain) and the gut-mitochondria axis (the connection between gut microbes and your cells’ energy production). This axis functions as a unified system, where microtubules, tiny structures within intestinal epithelial cells (IECs) and neurons, act like tracks to move mitochondria (the cell’s energy powerhouses) to where they are needed most, such as areas that support gut barriers or brain signaling. They also help transport signaling molecules, such as those involved in brain communication or gut health. While microtubules support the functions of these cells, they do not directly connect the gut and brain across the body. Instead, chemical messengers, such as short-chain fatty acids (SCFAs, including butyrate), help cells function properly, and bioelectrical signals, like those from the vagus nerve, convey messages between the gut and brain, thereby tying the whole axis together.
The Gut-Brain Axis: Your Gut Talks to Your Mind
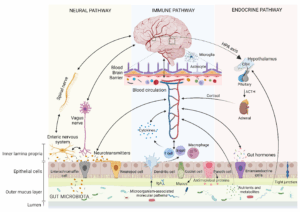
The gut-brain axis is a two-way communication highway, mediated by the vagus nerve, immune signals (e.g., cytokines), and microbial metabolites like SCFAs. Butyrate, a key SCFA, promotes serotonin production in the gut and supports conditions for gamma-aminobutyric acid (GABA) synthesis, influencing mood and neural calm. These neurotransmitters are transported in vesicles via microtubules within neurons and signaled through bioelectrical impulses, linking gut health to brain function. A balanced microbiome, fostered by high-fiber diets, exercise, and sunlight exposure, responds to positive natural cues, enhancing emotional stability and cognitive clarity.
Dysbiosis, however, disrupts this balance, reducing beneficial metabolites and increasing harmful ones like lipopolysaccharide (LPS), a toxin from gram-negative bacteria. This weakens the gut’s protective barrier, leading to a condition known as a “leaky gut.” Inflammatory molecules escape into the bloodstream, triggering neuroinflammation linked to depression, anxiety, and ASD. These disruptions are amplified by external cues, such as a diet rich in highly processed foods, a lack of physical activity, or exposure to artificial light at night (ALAN), which pulls the body away from its natural design.
The Gut-Mitochondria Axis: Powering Your Cells
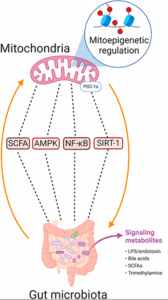
The gut-mitochondria axis governs the interplay between gut microbes and mitochondria, which produce ATP, the energy currency for high-demand cells like IECs and neurons. Butyrate enhances mitochondrial ATP production, stimulates biogenesis (new mitochondria formation), and reduces reactive oxygen species (ROS), supporting gut and brain health. Mitochondria, in turn, maintain gut barrier integrity by powering IECs. This axis is regulated by molecular connections—microtubules transport mitochondria to energy-demand sites, chemical messengers like SCFAs signal metabolic needs, and bioelectrical signals coordinate cellular responses. Positive cues, such as a polyphenol-rich diet or sunlight-driven circadian alignment, optimize this axis by boosting SCFA production and mitochondrial efficiency.
Dysbiosis disrupts this harmony, reducing SCFA production and increasing proinflammatory metabolites like LPS. This stresses mitochondria, elevates reactive oxygen species (ROS), reduces ATP production, and impairs intestinal epithelial cell (IEC) and neuronal function. Systemic inflammation, driven by circulating cytokines and reduced SCFA signaling, further compromises neuronal mitochondria, disrupting synaptic function and resilience. Negative cues, such as inadequate rest or dehydration, exacerbate these effects, driving the body toward disease.
The Bridge: Dysbiosis and Mitochondrial Stress Connect the Axes
The gut-brain and gut-mitochondria axes converge in the gut-mitochondria-brain axis, where dysbiosis-induced reductions in butyrate impair mitochondrial function in IECs and neurons, disrupting gut-brain signaling. This creates a vicious cycle: a leaky gut fuels systemic inflammation, which stresses neuronal mitochondria and reduces ATP, thereby amplifying neuroinflammation. Microtubules, chemical messengers, and bioelectrical signals propagate these disruptions across the axis, while external cues, like poor diet or lack of sunlight, exacerbate the imbalance. The result is a brain struggling to maintain balance, contributing to mental health and neurological conditions. Let’s explore how this bridge influences specific conditions:
- Depression and Anxiety: A 2022 study in Neuroscience Letters shows that mitochondrial dysfunction reduces serotonin signaling and increases neuroinflammation, key drivers of mood disorders. A dysbiotic gut, low in butyrate, exacerbates this by weakening mitochondrial support in neurons and IECs, thereby amplifying inflammation via the gut-brain axis. Negative cues like chronic stress or artificial light exposure disrupt bioelectrical signaling, further impairing mood regulation.
- Autism Spectrum Disorder (ASD): A 2023 study in the Journal of Behavioral Science found that individuals with ASD often have gut dysbiosis and elevated ROS, causing mitochondrial dysfunction and neural inflammation. Reduced SCFA production impairs neuronal energy metabolism, which may contribute to developmental challenges. Lack of movement or poor hydration may compound these effects by misaligning the axis with natural design.
- Neurodegenerative Diseases: In Alzheimer’s and Parkinson’s, mitochondrial dysfunction, oxidative stress, and gut dysbiosis are shared features, as noted in a 2024 article in Molecules. Gut-derived inflammatory molecules and amyloid-like proteins overburden neuronal mitochondria, accelerating brain cell damage. Deviations from natural cues, such as inadequate sleep or a processed diet, exacerbate this cycle.
A New Path Forward: Aligning with Natural Design
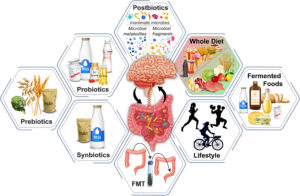
The gut-mitochondria-brain axis offers a pathway to restore health by aligning with our natural design through intentional interaction with the natural world. By targeting the microbiome and supporting mitochondrial function, we can restore gut-brain harmony and optimize whole-body health. Here are evidence-based strategies rooted in natural cues:
- Diet: High-fiber foods (vegetables, legumes [lentils], ancient grains) and polyphenol-rich foods (berries, walnuts) feed beneficial microbes, boosting SCFA production. A Mediterranean diet, rich in omega-3s, enhances mitochondrial health via microbial metabolites like urolithins, as shown in a 2024 study. Pure water supports cellular hydration, which is essential for mitochondrial function. Read more about water: The Fourth Phase of Water: Revolutionary Discoveries in Hydration Science and The biological impact of deuterium and therapeutic potential of deuterium-depleted water
- Sunlight: Responsible sunlight exposure regulates circadian clock rhythms, enhancing mitochondrial biogenesis and bioelectrical signaling via the vagus nerve. A 2023 study in Frontiers in Physiology highlights sunlight’s role in optimizing mitochondrial function and reducing oxidative stress.
- Movement: Exercise promotes gut microbial diversity and mitochondrial efficiency, as noted in a 2023 Biomedicines study. Physical activity enhances microtubule dynamics and bioelectrical signaling, supporting the axis. Read more: Exercise Upregulates Hsp60 and Supports Mitochondrial Function and Sun, Exercise, Reduced Sugar Intake Lowers All Cause Mortality
- Rest: Quality sleep helps restore mitochondrial function and gut barrier integrity, thereby reducing inflammation. A 2023 study in Sleep Medicine found a link between sleep and improved microbial balance and brain health.
- Probiotics and Prebiotics: These restore microbial balance, reducing inflammation and supporting mitochondrial function. A 2024 Heliyon study demonstrated that psychobiotics can reverse anxiety-like behaviors by optimizing the gut-mitochondria-brain axis.
A 2023 study in Behavioral Sciences found that fecal microbiota transplantation in children with ASD reduced gastrointestinal symptoms by nearly 80% and improved behavior, with lasting increases in beneficial bacteria like Bifidobacterium, underscoring the clinical potential of targeting this axis.
The Future of Health: A Holistic, Nature-Aligned Approach
The gut, mitochondria, and brain are not isolated—they form an interconnected axis driven by microtubules, chemical messengers, and bioelectrical signals, responding to cues from within and outside the body. When these cues align with our natural design—through sunlight, nutrient-dense diets, pure water, movement, and rest—the axis fosters health. Dysbiosis and mitochondrial stress, driven by poor cues like processed foods or lack of sleep, pull us toward disease. By embracing a holistic approach that targets the microbiome and mitochondria, we can optimize this axis and unlock vibrant health.
As research advances, we’ll uncover more ways to fine-tune the gut-mitochondria-brain network. For now, nurture your body with a high-fiber diet, fermented foods, and a lifestyle rich in natural cues: routine exercise, restorative sleep, stress management, responsible sun exposure, and pure water. These empower your gut, mitochondria, and brain, aligning your body with its natural design. Have you explored ways to boost your gut health or reconnect with nature? Share your thoughts, and let’s keep the conversation going!