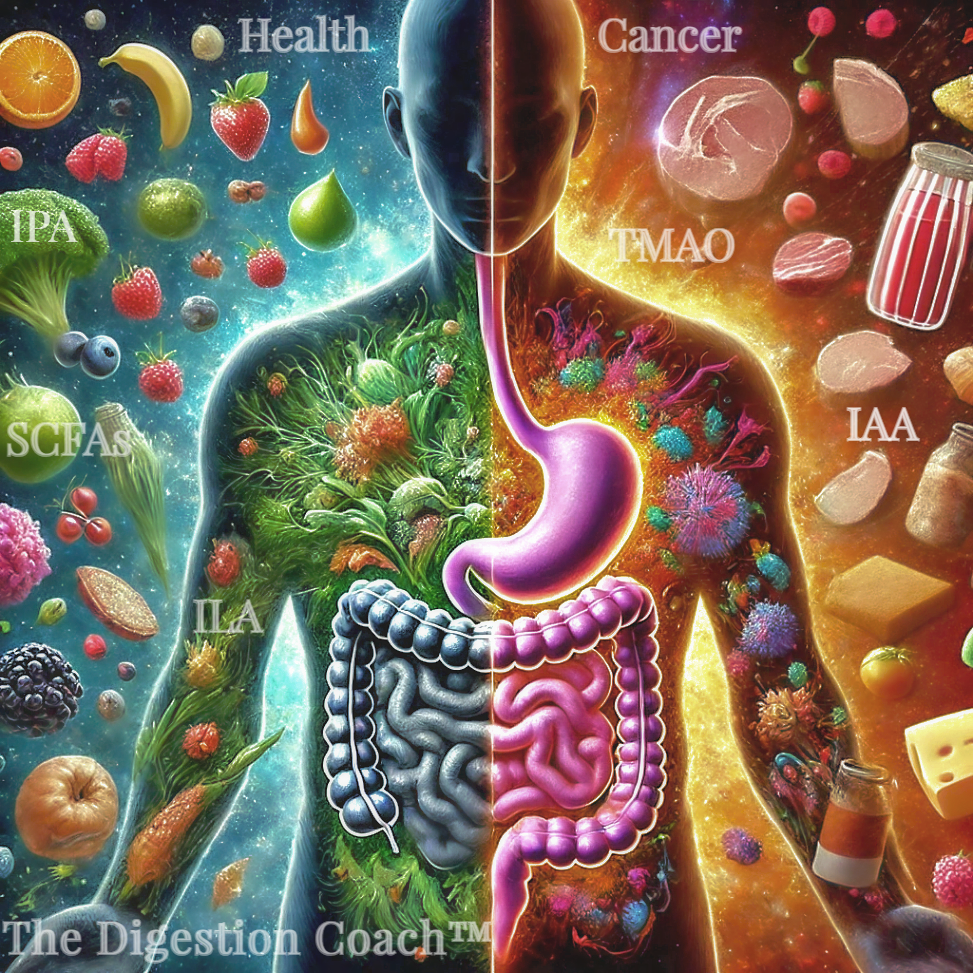
Is There a Choline, Carnitine, Tryptophan Cancer Risk via Diet? Trimethylamine N-oxide (TMAO) and indole-3-acetic acid (IAA) are metabolites produced by gut microbiota from specific dietary precursors found in certain foods. TMAO production occurs from the metabolism of nutrients containing trimethylamine groups, such as choline, carnitine, and betaine. These precursors are abundant in:
- Red meat (e.g., beef, lamb, pork) is rich in L-carnitine.
- Eggs, particularly the yolks, are high in choline.
- Fish (especially saltwater species like cod or shrimp) can contain trimethylamine or its precursors.
- Dairy products (e.g., milk, cheese) provide choline.
- Liver (e.g., from beef or chicken) is another choline source.
Gut bacteria convert these compounds into trimethylamine (TMA), which the liver then oxidizes into TMAO. The amount of TMAO produced depends on diet, gut microbiome composition, and individual metabolism.
IAA is a metabolite of tryptophan, an essential amino acid found in many protein-rich foods. Dietary sources of tryptophan that can lead to IAA production include:
- Poultry (e.g., turkey, chicken).
- Fish (e.g., salmon, tuna).
- Dairy (e.g., milk, yogurt, cheese).
- Eggs.
- Soy products (e.g., tofu, soybeans).
- Nuts and seeds (e.g., pumpkin seeds, walnuts).
- Legumes (e.g., lentils, chickpeas).
Once consumed, tryptophan is metabolized by certain gut bacteria (e.g., Clostridium or Bacteroides species) into indole derivatives, including IAA. The process hinges on both dietary intake and the specific microbial population in the gut.
In short, TMAO comes from foods rich in choline and L-carnitine (like red meat and fish), while IAA stems from tryptophan-rich foods (like poultry and dairy). Your diet and gut bugs decide how much of each gets made.
Trimethylamine N-oxide (TMAO) and Indole-3-Acetic Acid (IAA) influence disease and cancer progression by impacting tumor angiogenesis and immune modulation.
Research suggests that Trimethylamine N-oxide (TMAO) and indole-3-acetic acid (IAA) can influence disease and cancer progression through mechanisms like tumor angiogenesis and immune modulation. However, the details and extent of their roles are still being explored, and both, under a homeostatic setting, possess beneficial aspects as well.
TMAO, a gut microbiota-derived metabolite, has been implicated in various diseases, including cancer. Studies indicate that TMAO can promote angiogenesis—the formation of new blood vessels—which tumors often exploit to sustain growth. For example, in some preclinical models, TMAO has been shown to enhance vascular endothelial growth factor (VEGF) signaling, a key driver of angiogenesis. Additionally, TMAO may affect immune modulation by altering inflammatory responses or immune cell activity, potentially creating a tumor-friendly microenvironment. However, the exact mechanisms and whether TMAO universally promotes or inhibits cancer progression can depend on the cancer type (colorectal vs triple-negative breast cancer) and context.
IAA, another microbial metabolite derived from tryptophan, also appears to play a dual role in cancer. It can influence angiogenesis by interacting with pathways like the aryl hydrocarbon receptor (AhR), which regulates vascular development and immune responses. IAA’s immune-modulatory effects are notable too—under detrimental circumstances—like gut dysbiosis from low-fiber, high-protein diets or excessive tryptophan intake, IAA may suppress anti-tumor immunity by promoting regulatory T-cell activity or altering cytokine profiles. This may favor disorders like inflammatory conditions, neuropsychiatric diseases, metabolic syndromes, and cancer like colorectal or liver. However, IAA can also exhibit anti-inflammatory properties that might counteract disease progression under specific conditions (e.g., high fiber polyphenol-rich diet).
TMAO and IAA research is evolving. However, a comprehensive look with human data is needed to establish better mechanistic clarity regarding when and how these compounds can move from beneficial to harmful and vice versa. Below, I want to explore a line of thought that may help to bring out the better side of these two compounds.
- Gut microbiota in cancer: From molecular mechanisms to precision medicine applications
- Trimethylamine N-Oxide Promotes Cell Proliferation and Angiogenesis in Colorectal Cancer
- The gut microbiota derived metabolite trimethylamine N-oxide: Its important role in cancer and other diseases
- 3-indoleacetic acid (3-IAA) – Exploring its impact on human health and metabolism
Given that choline, carnitine, and tryptophan-rich foods have been consumed for millennia and are chemical backbones to critical bodily functions and structures, we must ask, what dietary or lifestyle changes have caused these foods to go from benign to potentially cancer-causing?
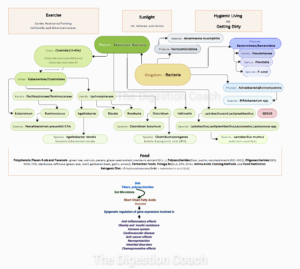
The shift from viewing choline, carnitine, and tryptophan-rich foods as benign—or even essential—to potentially cancer-causing isn’t about the foods themselves changing. Instead, it’s tied to how modern diets, lifestyles, and environments interact with our biology, particularly our gut microbiome, in ways that differ from our ancestors’ experiences. Here’s what’s likely at play:
1. Dietary Excess and Imbalance
Historical Context: For millennia, humans consumed choline (from eggs, fish), carnitine (from red meat), and tryptophan (from proteins like poultry) in moderation, often limited by availability and balanced with fiber-rich plants. Food scarcity and physical demands kept intake in check.
Modern Shift: Industrial agriculture has made these foods—like red meat (high in carnitine and choline), eggs (choline), and dairy (tryptophan)—cheap and abundant. We eat them more often, often processed (e.g., burgers, sausages), and with less fiber from fruits, vegetables, and whole grains. This floods the gut with carnitine and choline, spiking TMAO production—linked to angiogenesis and inflammation in cancer—while excess tryptophan may tilt toward IAA in an imbalanced microbiome.
2. Gut Microbiome Changes
Historical Context: Pre-industrial diets, rich in diverse plants and fermented foods, fostered a varied gut microbiome. This likely kept TMAO and IAA-producing bacteria in balance with other species.
Modern Shift: Antibiotics, sanitized environments, and low-fiber, high-fat diets have reshaped our gut flora. Bacteria like Clostridium and Proteobacteria now thrive on carnitine and choline, converting them to trimethylamine (TMA), which the liver oxidizes into TMAO. Similarly, tryptophan-metabolizing bacteria (e.g., Bacteroides) may favor indole-3-acetic acid (IAA) over protective indoles like indole-3-propionic acid (IPA) and indole-3-lactic acid (ILA). In ancestral guts, fiber-rich diets supported Lactobacillus and Bifidobacteria, boosting ILA and IPA for gut health, but these modern shifts amplify metabolites like TMAO and IAA, potentially tied to disease and cancer progression.
3. Sedentary Lifestyle and Inflammation
Historical Context: Active lifestyles and periodic fasting kept metabolic and inflammatory processes regulated. Choline, carnitine, and tryptophan metabolites were processed in a system tuned for efficiency.
Modern Shift: Sedentary habits and constant food availability lead to chronic inflammation and metabolic overload. TMAO—from carnitine and choline—may amplify this, promoting disease and tumor-friendly inflammation or angiogenesis. IAA, from tryptophan, might suppress anti-tumor immunity in this context, though it can also be anti-inflammatory elsewhere. The body’s handling of these metabolites is now misaligned from its evolutionary design.
4. Environmental and Industrial Factors
Historical Context: Food and metabolism faced fewer external stressors without industrial pollutants or synthetic additives. For example, more subtle cooking methods such as braising or stewing of meats were more common than today, where BBQ is now the trend.
Modern Shift: Pesticides, herbicides (e.g., glyphosate), preservatives, processed meats, non-stick pans containing polytetrafluoroethylene (PTFE), and high-heat cooking (e.g., grilling carnitine-rich steaks) introduce compounds that stress the gut and liver. Grilled meats may form carcinogens, such as polycyclic aromatic hydrocarbons (PAHs) and heterocyclic amines (HCAs), possibly amplifying TMAO’s effects. These modern twists on ancestral practices could enhance the cancer risk from choline, carnitine, and tryptophan metabolites.
5. Quantity and Context Matter
Historical Context: For centuries, choline (essential for cell membranes and neurotransmitters), carnitine (key for fat metabolism), and tryptophan (needed for serotonin and proteins) were consumed in moderation as part of a balanced diet. Our ancestors ate these nutrients—found in foods like meat, eggs, and fish—alongside polyphenol-rich spices (e.g., turmeric, garlic), high-fiber plants (e.g., grains, legumes), and fermented foods (e.g., yogurt, kraut). This combination, paired with an active lifestyle, kept their intake and metabolism in harmony, minimizing any health risks.
Modern Shift: These nutrients remain vital, but today’s habits push them to excess. Frequent red meat delivers 1-2g of carnitine, daily eggs load up on choline, and constant protein intake boosts tryptophan—all overloading a gut lacking the fiber and diversity of ancestral diets. Without enough fiber, carnitine, and choline-rich foods spike TMAO levels, while a disrupted microbiome may favor IAA from tryptophan. The potential cancer link isn’t the nutrients themselves—it’s how modern overconsumption and poor dietary balance skew their metabolism into risky territory.
Why Now?
These foods haven’t turned toxic; our relationship with them has changed. Overconsumption of carnitine and choline-rich red meat, alongside tryptophan sources, in a low-fiber, antibiotic-shaped gut, plus sedentary, inflammatory lifestyles, amplifies TMAO and IAA in ways evolution didn’t anticipate. Research—primarily associative from animal studies and human cohorts—suggests this tips a neutral system into risky territory, though causation remains debated. Context is everything.
Could adding to one’s diet or maintaining a higher ratio of insoluble fiber, soluble fiber, and fermented foods offset any negative impact derived from a Choline, carnitine, and tryptophan-rich diet?
1. Insoluble Fiber
- Mechanism: Insoluble fiber (e.g., cellulose from whole grains, vegetables like celery, or bran) speeds up gut transit time and bulks up stool. This reduces the time gut bacteria have to ferment choline into trimethylamine (TMA), the precursor to TMAO. Less TMA production could mean lower TMAO levels, potentially dialing back its pro-angiogenic or inflammatory effects linked to disease and cancer.
- Bonus: It also spreads out bacterial activity in the colon by adding bulk, which may reduce the dominance of TMAO-producing bacteria (e.g., certain Firmicutes) and encourage a gut environment with less TMA production.
2. Soluble Fiber
- Mechanism: Soluble fiber (e.g., beta-glucan from oats, inulin from lentils, pectin from apples) feeds beneficial gut bacteria like Bifidobacteria and Lactobacilli, which produce short-chain fatty acids (SCFAs) such as butyrate. SCFAs are anti-inflammatory and may counteract TMAO’s inflammatory effects. They also strengthen the gut barrier, reducing systemic inflammation that could amplify cancer risk.
- Tryptophan Tie-In: Soluble fiber might shift tryptophan metabolism away from indole derivatives like IAA, produced by Bacteroides in dysbiotic guts, toward pathways favored by fiber-fermenting bacteria (e.g., Clostridium sporogenes, Lactobacillus), yielding protective metabolites like IPA and ILA with more apparent anti-cancer benefits.
3. Fermented Foods
- Mechanism: Foods like yogurt, kimchi, or sauerkraut introduce probiotics and bioactive compounds that diversify the gut microbiome. A richer microbial community could outcompete TMAO-producing bacteria, lowering TMAO output. For instance, Lactobacilli species don’t typically churn out TMA, unlike some Clostridia.
- IAA Angle: Fermented foods might also nudge tryptophan metabolism toward protective indole compounds (e.g., indole-3-propionic acid and indole-3-lactic acid) rather than IAA, depending on the bacterial mix. This could tilt immune modulation away from tumor-friendly suppression.
4. Historical Perspective
- Ancestral Balance: Historically, diets across Europe (e.g., rye bread, sauerkraut), Asia (e.g., miso, rice bran), and Africa (e.g., fermented millet, sorghum) paired choline, carnitine, and tryptophan-rich foods—like meat, fish, and eggs—with spices (e.g., turmeric, garlic), high-fiber plants, and fermented staples. Cancer rates were far lower over a hundred years ago, likely due to this balance, active lifestyles, and less processed food, suggesting we must return to that holistic way of eating and living to mitigate modern risks.
The Big Picture
- TMAO Reduction: Studies show that high-fiber diets—especially with prebiotics such as inulin and fructooligosaccharides—cut TMAO levels by promoting the growth of bacteria that do not produce TMA. Adding fermented foods could amplify this by introducing microbes that don’t favor TMA production.
- IAA Modulation: The effect on IAA is murkier, but a fiber and fermentation-rich diet might diversify tryptophan metabolites, potentially reducing cancer-linked immune suppression and boosting anti-inflammatory benefits.
- Balance Restored: Historically, humans ate choline, carnitine, and tryptophan-rich foods alongside heaps of fiber and naturally fermented stuff. Reintroducing that balance could mimic an ancestral gut environment, where these metabolites didn’t seem to pose the same risks.
Caveats
- Dose and Timing: You’d need enough fiber (say, 20-30g daily, split between soluble and insoluble) and consistent fermented food intake to see an effect. A one-off salad won’t cut it against a steak-heavy diet.
- Individual Variation: Gut microbiomes differ wildly. If yours is already skewed toward TMAO-producers, it might take time—or even specific strains (e.g., via probiotics)—to shift the balance.
- Cancer Link Strength: The TMAO/IAA-cancer connection via a choline, carnitine, and tryptophan-rich diet isn’t entirely proven in humans; it’s stronger in mice and associative in people. So, while fiber and fermentation might lower these metabolites, the cancer risk reduction is still hypothetical.
Verdict
In theory, piling on fiber and fermented foods could counteract the downsides of a choline, carnitine, and tryptophan-rich diet by tweaking gut metabolism and inflammation. It’s a plausible reset to a more “ancestral” system, and there’s decent evidence for TMAO reduction. For IAA, it’s less clear but still promising. Practically, it’s a low-risk move—fiber and fermentation are already health winners—so it’s a solid bet even if the cancer angle remains a work in progress.
Theoretically, to reduce cancer risk, curb TMAO producers and support a higher ratio of Indol-3-propionic acid (IPA) and Indole-3-lactic acid good guys vs Indol-3-acetic acid (IAA) good guy/bad guy. We should consume 20-30 grams of fermented, insoluble, and soluble fiber-dense foods daily. Here is an example of what a daily dose of some of the most researched foods for this purpose look like (broccoli, lentils, oat bran, apple pectin, sauerkraut, and chia seeds).
Theoretically, to reduce cancer risk, curb TMAO producers, and support a higher ratio of indole-3-propionic acid (IPA) and indole-3-lactic acid (ILA)—gut-derived tryptophan metabolites with anti-inflammatory and protective properties—over indole-3-acetic acid (IAA), which may promote tumor-friendly inflammation, consuming 20-30 grams daily of fermented, insoluble, and soluble fiber-dense foods could help. A higher IPA-to-IAA ratio is beneficial because IPA may lower cancer risk by reducing inflammation and supporting gut health. At the same time, IAA’s potential to enhance angiogenesis and immune suppression could worsen cancer outcomes. Here’s an example of a daily dose using well-researched foods:
To hit 20-30 grams of daily fiber using broccoli, lentils, oat bran, apple pectin, sauerkraut, and chia seeds—here’s a practical plan optimized for fiber content, fermentation benefits, and microbiome effects. These foods nourish beneficial bacteria like Bacteroides vulgatus, Bifidobacteria, Lactobacillus, Clostridium sporogenes, Akkermansia muciniphila, Faecalibacterium prausnitzii, Roseburia, and Eubacterium, supporting your goals. Portions are based on typical nutritional data, balancing insoluble, soluble, and fermented elements.
Breakfast: Oat Bran and Chia Porridge
- 1/2 cup dry oat bran (soluble + insoluble): ~7g fiber (beta-glucan feeds Bifidobacteria and Roseburia, boosting SCFAs like butyrate while diluting TMAO production; supports ILA via Bifidobacteria).
- 1.5 tbsp chia seeds (soluble + insoluble): ~7.5g fiber (mucilage supports B. vulgatus and F. prausnitzii, enhancing gut diversity and IPA/ILA pathways).
- Total: ~14.5g fiber. (Cook oat bran in water, stir in chia, and let it thicken.)
Snack: Apple Pectin and Sauerkraut
- 1 tbsp apple pectin powder (soluble): ~3g fiber (pectin ferments into SCFAs via B. vulgatus and Eubacterium, supports Akkermansia, and may enhance IPA/ILA production).
- 1/4 cup sauerkraut (fermented + insoluble): ~1g fiber (Lactobacillus plantarum diversifies the gut, counters TMAO producers, and pairs with indoles for IPA via Clostridium sporogenes and ILA via Lactobacillus).
- Total: ~4g fiber. (Mix pectin into water; eat sauerkraut on the side.)
Lunch: Lentil and Broccoli Bowl
- 1/2 cup cooked lentils (soluble + insoluble): ~4g fiber (inulin and resistant starch feed F. prausnitzii and B. vulgatus, lowering TMAO; tryptophan supports IPA via Clostridium sporogenes and ILA via Bifidobacteria).
- 1 cup steamed broccoli (insoluble + soluble): ~3g fiber (glucosinolates provide indole precursors for Clostridium sporogenes to favor IPA and Lactobacillus for ILA over IAA; fiber boosts Roseburia for butyrate).
- Total: ~7g fiber.
Daily Breakdown
Fiber Total: 14.5 (breakfast) + 4 (snack) + 7 (lunch) = 25.5g. Fits the 20-30g range. Adjust to ~23g (1 tbsp chia, 5g fiber) or ~27.5g (3/4 cup lentils, 6g fiber) as needed.
- Fermented: Sauerkraut’s Lactobacillus plantarum fosters diversity, aiding F. prausnitzii and Roseburia for butyrate and Clostridium sporogenes for IPA, plus ILA production.
- Soluble Fiber: Oat bran (beta-glucan), apple pectin (pectin), chia (mucilage), and lentils (inulin) fuel Bifidobacteria, B. vulgatus, and butyrate producers (Roseburia, Eubacterium).
- Insoluble Fiber: Broccoli, lentils, oat bran, and sauerkraut speed transit, reducing TMAO and supporting SCFA delivery.
Why This Works?
- TMAO Reduction: High fiber from lentils, oat bran, and chia curbs TMA synthesis by feeding non-TMAO producers like Bifidobacteria, Bacteroides vulgatus, and Faecalibacterium prausnitzii. Sauerkraut’s Lactobacillus outcompetes TMA producers (e.g., some Clostridia). TMAO reduction decreases choline, carnitine, and tryptophan cancer risk.
- IPA/ILA vs. IAA: Broccoli’s glucosinolates and lentils’ tryptophan boost IPA via C. sporogenes and ILA via Lactobacillus and Bifidobacteria, while B. vulgatus may produce some IAA. Diverse fiber supports a gut favoring IPA and ILA over IAA.
- Butyrate Boost: Oat bran, lentils, and broccoli feed Roseburia, F. prausnitzii, and Eubacterium for butyrate, enhancing anti-cancer effects alongside B. vulgatus’s acetate/propionate.
- Research Backing: In microbiome studies, oat bran and lentils boost SCFAs (including butyrate). Broccoli’s indoles support IPA and ILA. Pectin and chia enhance Bacteroides and butyrate producers. Sauerkraut’s fermentation benefits are well-documented.
Practical Notes
Fiber: 14.5 (breakfast) + 4 (snack) + 7 (lunch) = 25.5g
Protein: 11 + 0.5 + 12 = 23.5g
Fat: 8.5 + 0 + 1 = 9.5g
Total Carbs: 38.5 + 5.5 + 26 = 70g
Net Carbs: 24 + 1.5 + 19 = 44.5g
Calories: ~500-550 kcal (oat bran ~160, chia ~90, pectin ~15, sauerkraut ~10, lentils ~115, broccoli ~55). Leaving room for other foods (e.g., carnitine, choline/tryptophan sources).
Hydration: Pair with 2-3 liters of water to keep fiber moving.
This lands at 25.5g fiber—with a mix optimized to curb TMAO and tilt the IPA: IAA ratio, all rooted in solid gut science.
Conclusion
In exploring how choline, carnitine, and tryptophan-rich foods—longtime staples of human diets—might now contribute to cancer via metabolites like TMAO and IAA, we’ve uncovered a story of context, not inherent harm. Research suggests these compounds influence tumor angiogenesis and immune modulation, but the evidence, while promising, remains inconclusive, rooted more in preclinical models than definitive human trials. Historically, across Europe, Asia, and Africa, these nutrient-dense foods were balanced with spices, fiber, and fermented goods, aligning with lower cancer rates over a century ago—a stark contrast to today’s overconsumption, low-fiber diets, and disrupted microbiomes that amplify TMAO and skew IAA production. Theoretically, reintroducing insoluble and soluble fiber—say, 20-30g daily from broccoli, lentils, oat bran, apple pectin, and chia seeds—alongside fermented foods like sauerkraut, could curb TMAO, favor protective IPA over IAA, and restore an ancestral gut balance. Cancer’s complexity and individual microbiome quirks mean it’s not a guaranteed fix. Still, it’s a low-risk, evidence-backed step toward reclaiming how we once ate and lived—before these vital foods turned into modern risks.
COMPLEMENTARY 15-MINUTE CALL
Take your first step toward a renewed sense of well-being. Call today to arrange a complimentary 15-minute consultation.
Let’s discern whether my approach aligns with your needs.
I look forward to connecting with you at 714-639-4360.